At Ultimate Treat, we’re passionate about understanding the science behind health and nutrition. The Ferric Reducing Antioxidant Power (FRAP) assay is a powerful tool in this field.
This method allows us to measure the antioxidant capacity of various substances, providing valuable insights into their potential health benefits. In this post, we’ll explore the principle behind the FRAP assay and its significance in antioxidant research.
What Are Antioxidants and Free Radicals?
The Battle Within Our Bodies
Free radicals are unstable atoms that can damage cells, causing illness and aging. Our bodies produce them naturally, but factors like pollution, UV radiation, and poor diet increase their numbers. Antioxidants act as the body’s defence, neutralizing these harmful free radicals.
The Importance of Antioxidants
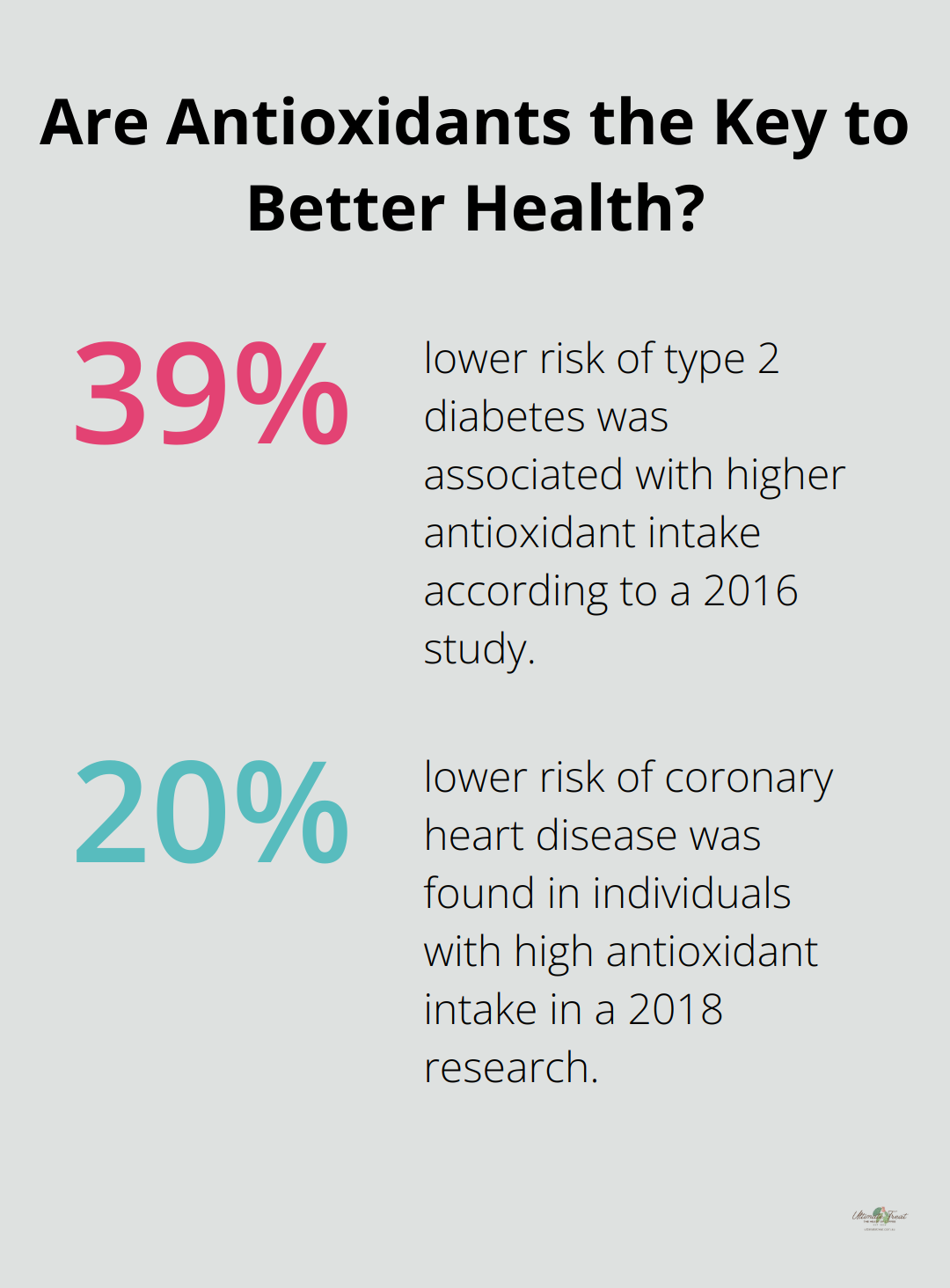
Antioxidants play a vital role in preventing and reducing oxidative stress, which can lead to various health issues. A study published in the Journal of Nutritional Science 2016 found that higher antioxidant intake was associated with a 39% lower risk of type 2 diabetes. Research from the American Journal of Clinical Nutrition in 2018 showed that individuals with high antioxidant intake had a 20% lower risk of coronary heart disease.
Boosting Your Antioxidant Intake
While supplements offer an option, a varied diet provides the best way to increase your antioxidant intake. Here are some top antioxidant-rich foods:
- Berries: Blueberries, strawberries, and raspberries pack a powerful antioxidant punch.
- Dark chocolate: A 100-gram bar of dark chocolate can contain up to 15,000 antioxidant units.
- Pecans: A one-ounce serving provides 5,095 antioxidant units.
- Artichokes: One medium artichoke offers 4,416 antioxidant units.
- Coffee: A typical cup of coffee contains about 1,299 antioxidant units.
The Power of Synergy
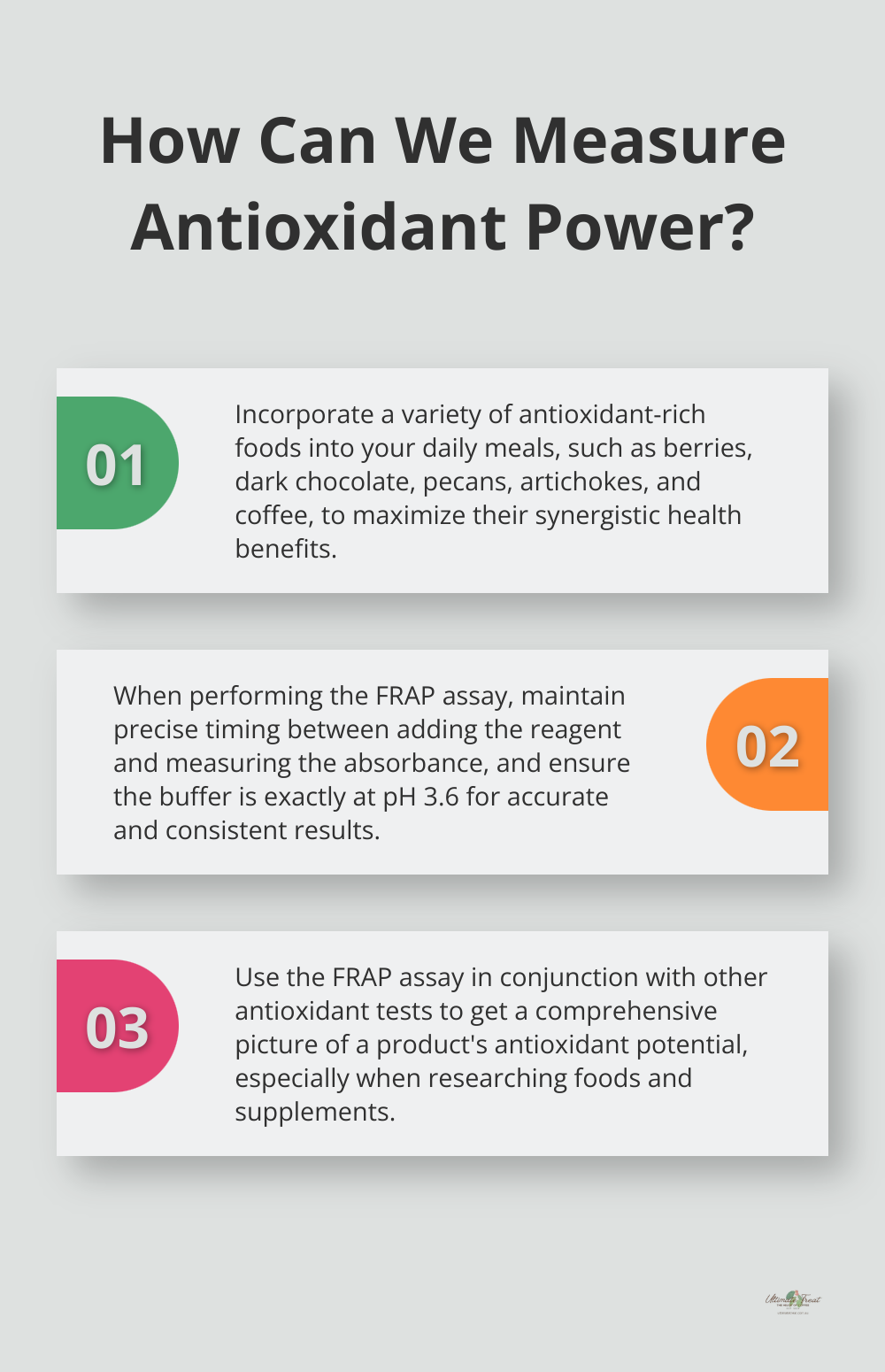
Antioxidants work best in combination. A diverse diet rich in fruits, vegetables, nuts, and even coffee provides various antioxidants that work synergistically to protect your health. Try to incorporate various foods into your daily meals to maximize their benefits.
Measuring Antioxidant Power
Scientists use various methods to measure the antioxidant capacity of different substances. One such method is the Ferric Reducing Antioxidant Power (FRAP) assay. This powerful tool allows researchers to quantify the antioxidant potential of various foods and supplements, providing valuable insights into their potential health benefits. In the next section, we’ll explore the principle behind the FRAP assay and its significance in antioxidant research.
How Does the FRAP Assay Work?
The Chemistry Behind FRAP
The Ferric Reducing Antioxidant Power (FRAP) assay is a cornerstone of antioxidant research. This method, developed by Benzie and Strain in 1996, quantifies the antioxidant capacity of various substances. The FRAP assay provides valuable insights into the potential health benefits of different foods and supplements.

The FRAP assay relies on a simple yet elegant chemical reaction. It measures the ability of antioxidants to reduce ferric ions (Fe3+) to ferrous ions (Fe2+) in an acidic environment (typically at a pH of 3.6). This assay’s key reagent is a ferric iron complex and 2,4,6-tripyridyl-s-triazine (TPTZ).
When an antioxidant is present, it donates electrons to the ferric-TPTZ complex, reducing it to a ferrous-tripyridyltriazine complex. This reduction causes a colour change from pale yellow to intense blue. The intensity of this blue colour (measured at 593 nm) directly correlates with the sample’s antioxidant capacity.
Practical Applications of FRAP
The FRAP assay has wide-ranging applications in food science, nutrition, and health research. For instance, a 2002 study published in the Journal of Agricultural and Food Chemistry used it to compare the antioxidant capacity of various fruits and vegetables. The researchers found that strawberries and spinach had exceptionally high FRAP values, which indicated strong antioxidant potential.
In the beverage industry, scientists use the FRAP assay to evaluate the antioxidant capacity of different types of tea and coffee. A 2003 Journal of Agricultural and Food Chemistry study found that green tea had the highest FRAP values among various tea types, followed by black and herbal tea.
Limitations and Considerations
While the FRAP assay is a powerful tool, it’s important to note its limitations. The assay primarily measures the ability of antioxidants to donate electrons, which doesn’t capture all types of antioxidant activity. For example, it may underestimate the antioxidant capacity of substances that work primarily through other mechanisms (such as carotenoids).
Moreover, scientists conduct the FRAP assay at a non-physiological pH, which may not accurately reflect antioxidant activity in the human body. Therefore, researchers often use FRAP with other antioxidant assays to better understand antioxidant capacity.
Despite these limitations, the FRAP assay remains a valuable tool in antioxidant research. Its simplicity, speed, and cost-effectiveness make it an attractive option for researchers and food scientists.
The Future of FRAP
As research in antioxidants continues to evolve, so does the application of the FRAP assay. Scientists are exploring ways to enhance its accuracy and broaden its scope. Some researchers are investigating the use of alternative ligands to TPTZ, which could potentially capture a broader range of antioxidant activities.
Others are working on adapting the FRAP assay for high-throughput screening, allowing for rapid testing of large samples. This could prove particularly useful in the food and supplement industries, where quick and reliable antioxidant testing is in high demand.
The next step in exploring the FRAP assay involves examining how researchers perform this test in the laboratory. Understanding the practical aspects of the FRAP assay will provide a more complete picture of its role in antioxidant research.
How to Perform the FRAP Assay
Equipment and Reagents
The Ferric Reducing Antioxidant Power (FRAP) assay requires specific equipment and reagents. Essential items include a spectrophotometer (capable of measuring absorbance at 593 nm), a water bath or heating block (set to 37°C), and precision pipettes.
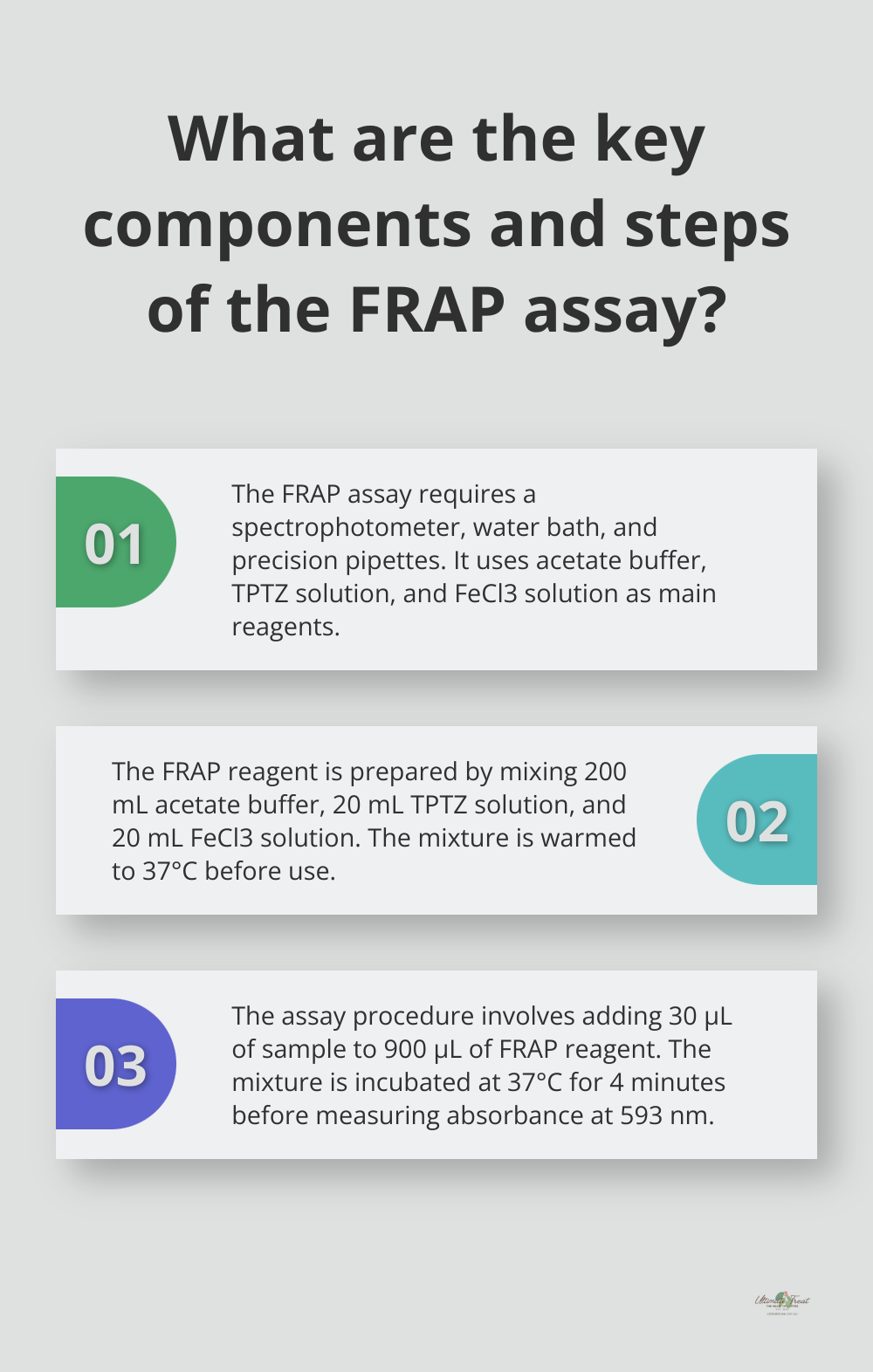
The primary reagents required are:
- Acetate buffer (300 mM, pH 3.6)
- TPTZ (2,4,6-tripyridyl-s-triazine) solution (10 mM in 40 mM HCl)
- FeCl3 solution (20 mM)
- Trolox standard solutions (100-1000 µM)
Assay Preparation
Prepare the FRAP reagent by mixing 200 mL of acetate buffer (pH 3.6), 20 mL of TPTZ solution, and 20 mL of FeCl3 solution. The reagent should be dark blue before adding the sample. Warm this mixture to 37°C before use.
Next, prepare your samples and standards. Dilute your antioxidant samples appropriately. For the Trolox standards, create a series of dilutions ranging from 100 to 1000 µM.
Assay Procedure
Add 30 µL of sample or standard to 900 µL of FRAP reagent to run the assay. Incubate this mixture at 37°C for precisely 4 minutes. After incubation, measure the absorbance at 593 nm using your spectrophotometer.
Result Interpretation
Express the FRAP value as µmol Trolox equivalents per gram or litre of sample. To calculate this, create a standard curve using the Trolox standards. Plot the absorbance values against the known Trolox concentrations.
Use this standard curve to determine the FRAP values of your samples. Higher FRAP values indicate greater antioxidant capacity. A study found that blueberries had FRAP values ranging from 33.03 to 73.71 μM Fe2+/g.
Practical Tips
Timing is critical when performing the FRAP assay. The reaction continues over time, so maintain consistent timing between adding the reagent and measuring the absorbance for accurate results.
The FRAP assay is sensitive to pH. Ensure your buffer is precisely at pH 3.6 to maintain consistency across experiments.
While the FRAP assay provides valuable data, it represents just one measure of antioxidant capacity. To comprehensively understand a product’s antioxidant potential, try combining FRAP results with other antioxidant assays (this approach is particularly useful in food and supplement research).
Final Thoughts
The Ferric Reducing Antioxidant Power (FRAP) assay principle provides a reliable method to quantify antioxidant capacity. This technique measures the ability of antioxidants to reduce ferric ions to ferrous ions, offering insights into the potential health benefits of various substances. The FRAP assay’s simplicity, speed, and cost-effectiveness make it an attractive option for researchers and food scientists worldwide.
Despite its advantages, the FRAP assay has limitations. It primarily measures electron-donating capacity, which may not capture all types of antioxidant activity. Non-physiological pH conditions might not accurately reflect antioxidant behaviour in the human body. Researchers continue to explore ways to enhance the accuracy and broaden the scope of this assay, including investigating alternative ligands and adapting it for high-throughput screening.
At Ultimate Treat, we recognise the importance of antioxidants in promoting overall health. Our premium organic coffee blend contains ingredients known for their high antioxidant content. We strive to provide a delicious and health-conscious coffee experience that aligns with the latest scientific understanding of antioxidants and their benefits.



